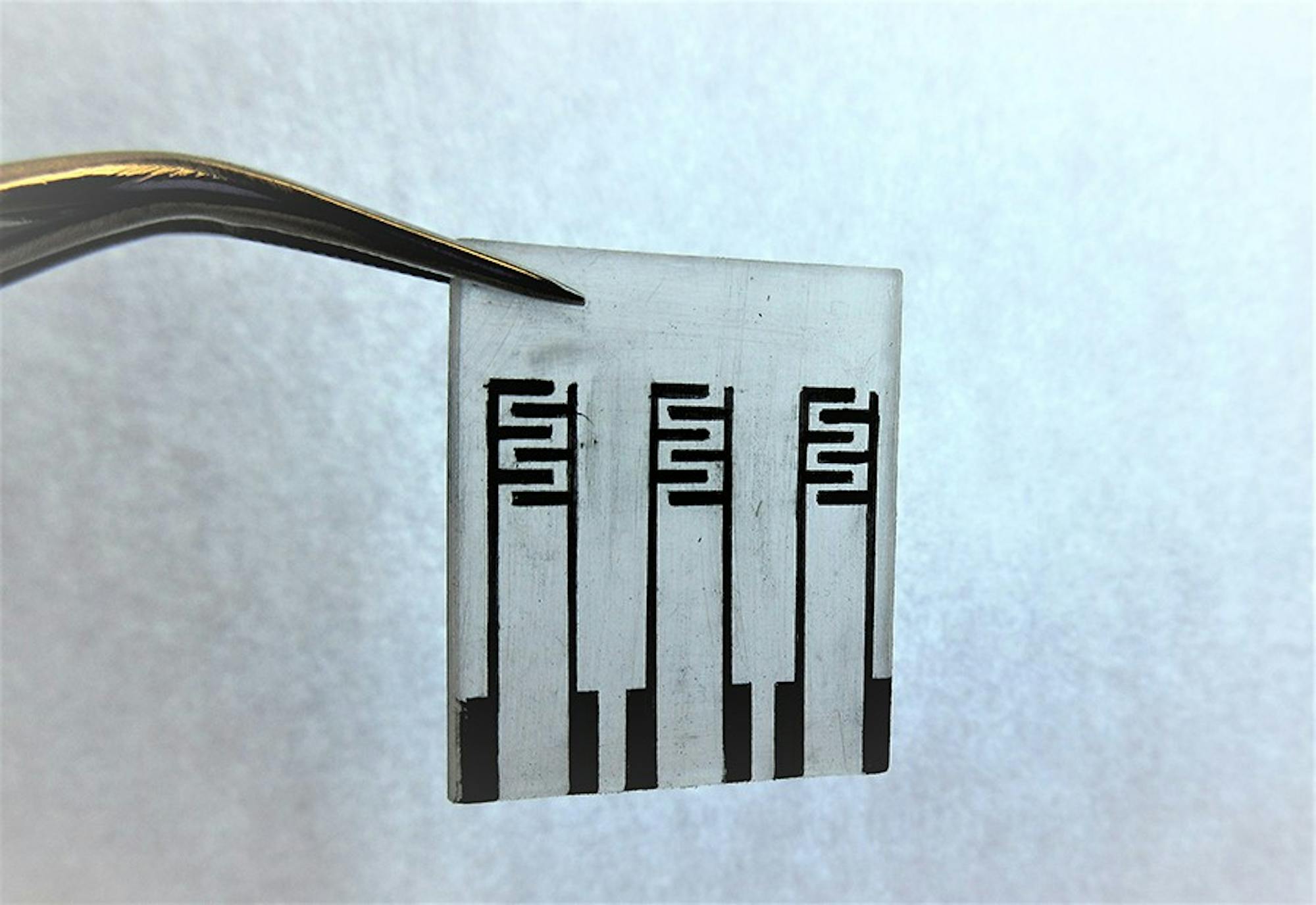
Using objects such as yellow wooden pencils and Shrinky Dinks, a child’s plastic toy that shrinks in size after being baked in an oven, chemistry professor Katherine Mirica and her team are developing a unique approach to build a portable and efficient electronic “nose,” a device to help detect toxic gases and environmental pollutants in the air and human bodies.
An expert on nanomaterials, Mirica found in previous work that there was no single technology available to detect and monitor the chemical identity of gases harmful to the environment or humans. Unlike other experiments, Mirica’s lab is unique in the unconventional materials her team uses. According to Mirica, the creation of a simple device that uses a sophisticated nanomaterial, such as this electronic nose, will allow researchers the capability to capture and detect gases.
Mirica noted that the initial design and paper published in July 2016 were just a start and that she expects the team to continue new research to further develop and enhance the product, potentially for commercial use.
“We are still too close to the fundamental science and there are many interesting questions that remain,” Mirica said. “Typically the path to commercialization works when the product is targeting a very specific goal. We are still in the stage where there [are] too many unknowns.”
Chemistry postdoctoral scholar Merry Smith, who works in Mirica’s lab, noted that the development of the “nose” provided solutions for two challenges the team had faced. The first challenge was difficulty transitioning insoluble materials from a lab setting to a functional device, necessitating the creation of a direct assembly method and the device’s simple shrinkable architecture. The second was designing a device that could be used with their metal organic frameworks and also had some capability to detect gases and pollutants.
Two potential solutions for the team were to either buy the electrodes from a company or create them in a lab, but the team wanted something that was inexpensive and fast. They found that the cheapest way was to take a graphite pencil, which conducts electricity and serves as a source of electrodes, to draw the sensors directly on to Shrinky Dinks.
The team hopes to create a portable device, similar to a breathalyzer, that can detect gases that thus far have not been easily detectable, such as ammonia, nitrogen oxide and hydrogen sulfide. The ability to detect and differentiate between these gases in a breath-based system would allow for breath-based diagnostics of disease recognition and monitoring in humans, Smith said.
Kennedy Jensen ’18, an author of the paper who was involved with the project, said she hopes to apply the technology in a medical setting.
“My work has been refocused on tuning and controlling the nanoscale morphology of the metal organic framework itself,” Jensen said. “Those different morphologies will have different applications [that] are really interesting because they are gasotransmitters, which means the body uses them in very small amounts for cardiac and neuro functioning and indicators for various illnesses like Alzheimer’s, Lou Gehrig’s disease, Parkinson’s disease.”
Kennedy said that she originally got involved in the research as a participant in the Women in Science Project, which shifted her perspective about the typical trajectory of research.
“I think overall there is a grand misconception of people outside the research world that research is linear,” Jensen said. “But the story you end up writing about in the end can be completely different than the question you set out to answer in the beginning. But that’s okay.”
Jensen added that she’s also learned to appreciate the journey of research, rather than the outcome.
“It’s not just about the end result, but the process of getting there,” Jensen said. “It’s not something I knew three years ago, but something I’m reminded of everyday in the lab.”
Chemistry graduate student Aylin Aykanat Med’19 expressed a similar sentiment about the process of research. Aykanat started at the lab approximately at the same time as Mirica, which she said was particularly promising because of the fresh cooperative dynamic and the ability to grow a project from ground up.
“Being a part of the collaborative lab environment not only allowed me to meet incredible people but helped me push the limits for myself as well,” Aykanat said. “Putting the work into it and seeing it come to fruition was incredible.”
Aykanat said the process taught her a lot about applied research and described the experience as motivating.
“Seeing something you make in a lab serve as a functional device to make lives better is incredible,” she said.
Since the paper was published last summer, Aykanat has continued developing new conductive materials to be made into gas sensors.
“We have to keep developing these new materials and see what they can do, what they can sense and how they will react with these different gaseous analytes,” Aykanat said.
Lebanon High School senior Polina Pivak joined the project back in summer 2015 as part of a program for high school students sponsored by the American Chemical Society. Pivak was initially unsure of the potential outcome of the project, but the moment when the device was exposed to vapors and responded, she felt that they had “come upon something important.”
“In chemistry labs in high school, you already know what the outcome or conclusion is going to be,” Pivak said. “The lab is actually just for learning the steps and seeing it for yourself, but working at the lab at Dartmouth, you don’t actually know what the answer is going to be or where your research is necessarily going to go.”
The lab also gave Mirica the opportunity to present her findings at the American Chemical Society National Conference.
Pivak said it was the creative aspect of the lab and the team’s ability to try anything new and different that allowed for interesting discussions with the team.
“It’s fascinating to feel as if you are on the brink of something,” Pivak said.
Paulomi is a junior from Connecticut and at Dartmouth she is an Economics and Government major with a public policy minor. Outside of The Dartmouth, Paulomi is involved with Women's Club Lacrosse, the Rockefeller Center for Public Policy, and Women in Business. Paulomi is excited to travel to Antarctica in winter 2018 to study public policy in the field.